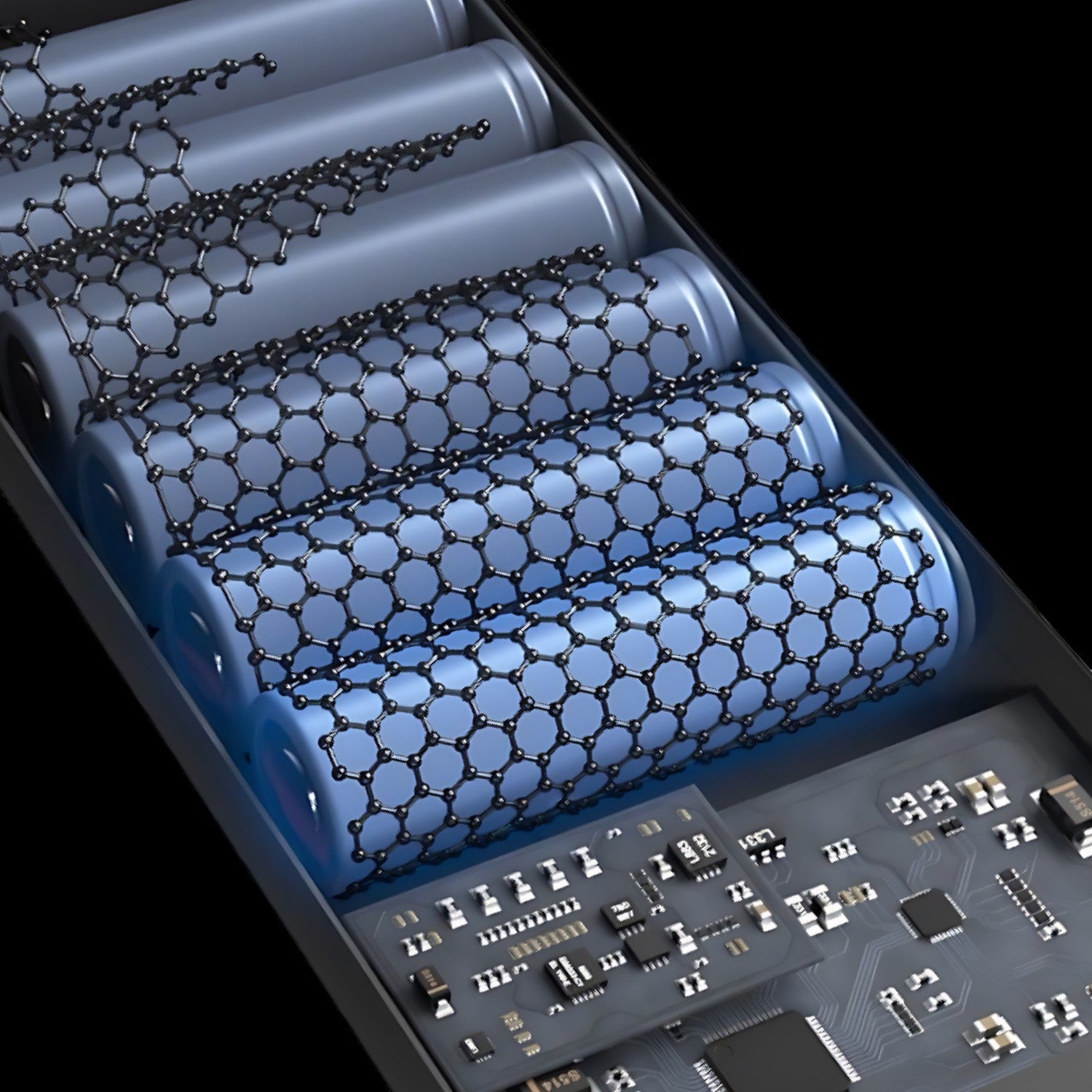
Graphene polymer batteries are a type of battery that uses graphene as the primary component of the electrodes, in combination with a polymer electrolyte. Graphene is a two-dimensional material made up of a single layer of carbon atoms arranged in a hexagonal lattice. It has a number of unique properties, including high conductivity, high strength, and high surface area, which make it an attractive material for use in batteries.
In a graphene polymer battery, the electrodes are made from a mixture of graphene and a polymer binder, which holds the graphene particles together and helps to improve the mechanical properties of the electrodes. The electrolyte is typically a polymer material that is highly conductive and allows ions to flow freely between the electrodes.
One of the main advantages of graphene polymer batteries is their high energy density, which means that they can store a lot of energy in a small amount of space. They also have the potential to be faster charging and have a longer lifespan than traditional batteries. However, they are still an emerging technology and there is ongoing research and development to optimize their performance and make them more practical for use in a variety of applications.

Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.